Providing solutions to support drug development
“With GMP management, we handle API and intermediate production from development to full-scale manufacturing.”
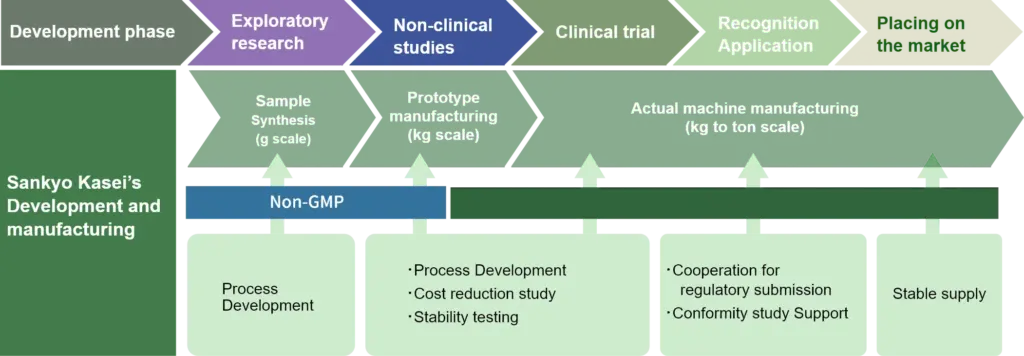
At the Fukuchiyama Works, we hold a pharmaceutical manufacturing license and manufacture a variety of active pharmaceutical ingredients and intermediates using a variety of equipment, including reaction vessels with capacities of up to 10,000 liters (including high-pressure and high-temperature/low-temperature reactors), filters, columns and dryers. Based on our past experience in developing new drugs, we can also support pharmaceutical development from the same perspective as pharmaceutical companies, including process development, manufacturing and, upon request, regulatory compliance measures such as master file registration.

“We support every phase from research to launch.”
Manufacture of major equipment

QC-LAB
Facility for conducting analysis and quality control in a class 100,000 clean room for active pharmaceutical ingredients and intermediates.

S-3
The S-3 is a dedicated plant for bulk pharmaceuticals and intermediates, capable of handling temperatures from -80°C to 250°C and a wide variety of synthesis such as GL, SUS, Hastelloy, autoclave, and clean room (Class 100,000).

Product warehouse
Intermediate and product storage areas with temperature control by area
(control temperature range: 0–30°C)
