Fukuchiyama Works Overview
cGMP certified factory
4 lines for Pharmaceuticals
Manufacturing Items
- ・APIs
- ・Pharmaceutical intermediates
Features
- ・Specialized facilities for APIs and intermediates

GMP manufacturing from gram to ton scale Low temperature reaction facilities (-80 ℃)
Column manufacturing for actual production High-temperature, high-pressure reaction facilities (160℃, 1MPa)

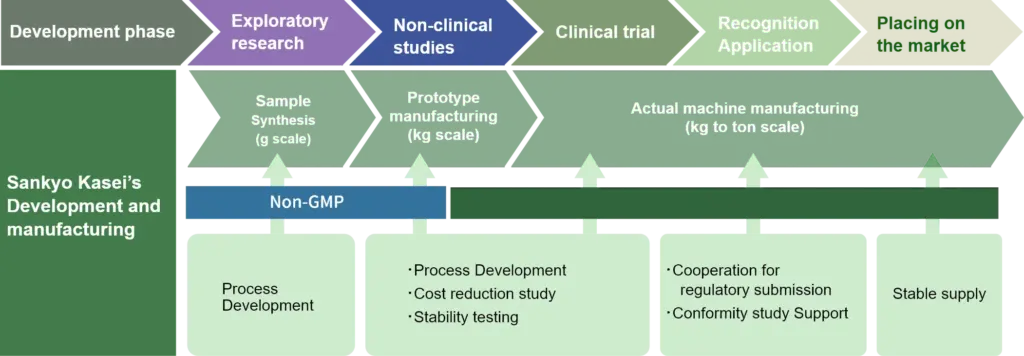
“We support every phase from research to launch.”
Equipment
Equipment
Material
Size
Number
Reactor
GL
50~10,000L
19
-none-
SUS
300~3,000L
6
-none-
Hasteloy
500
1
Column
SUS
700L
(600 mmφ × 2500 mm)
2
-none-
SUS
200L
(300 mmφ × 2500 mm)
1
Centrifuge
SUS
24~48inch
9
Filter
GL
600~900mmφ
3
-none-
SUS
500mmφ
1
Equipment
Material
Size
Number
Filter dryer
GL
1.5㎡
1
Dryer
SUS
Air blower type
100~400L
4
-none-
SUS
Mixing type 150L
2
-none-
SUS
Nauta dryer 3,000L
1
-none-
SUS
Shelf type
1
Crusher
SUS
Comil
1
-trans-
-trans-
-trans-
-trans-
-trans-
